According to the World Health Organisation, Campylobacter jejuni ranks as the top cause of food-borne bacterial diarrhoeal disease in the developed world, with chicken serving as the most common source of infection. While generally mild, C.jejuni infections can be fatal among the very young, elderly, and immunosuppressed individuals, a report by Dr Ceinwen Evans
At one time, C.jejuni was thought to be part of a normal microbial community in chickens. However, it is now understood that birds have a strong inflammatory response to C.jejuni. The infection causes diarrhoea, which can increase the risk of footpad lesions as the result of standing on wet litter.
In addition to improved processing plant sanitation, on-farm strategies – such as biosecurity measures and litter management best practices – can also be used to help reduce the risk of human pathogen exposure. Antibiotics have also proven effective in treating C.jejuni. However, the increasing pressure to reduce antibiotics in production has removed this treatment option in many cases. Overall, antibiotic reduction has opened the door to rising and unpredictable pathogen challenges, including C.jejuni, leading to poor flock performance and livability.
In today’s production environment, veterinarians have fewer weapons to fight disease challenges, live production managers are challenged to maintain consistency, and nutritionists are overwhelmed by the number of different feed options to test. It’s clear that the removal or reduction of antibiotics is driving fundamental change within animal production, creating new challenges that affect both animal and human health.
Search for alternatives
There has never been a more urgent need for a new approach to bridging animal nutrition and health with the demands of the consumer. Understanding the relationship between feed ingredients and compounds and their impact on the gut microflora is a crucial part of this approach, but it is proving to be a complex issue.
The global push to reduce antibiotics in recent years has resulted in a flood of antibiotic replacement solutions in the market. Prebiotics, probiotics, yeasts, essential oils, plant extracts, organic acids and other products all claim to solve a piece of the antibiotic-free puzzle. However, they often lack credible scientific evidence to support performance improvement claims or demonstrate an understanding of how they work in the gut.
In fact, when it comes to evaluating the impact of nutritional intervention on animal health, there remains significant room for improvement. Current tools for measuring the success of feed additives are still heavily reliant on traditional principles, such as Feed Conversion Ratios (FCR) and Average Daily Gain (ADG). In today’s changing environment, producers need reliable measurements that can take a versatile approach – addressing both external measurements as well as the state of the bird’s overall intestinal health and how it impacts health and performance.
Fresh approach to gut health
A new approach addresses the relationship between animal feed composition and a healthy gut function, which is known to be a crucial factor in determining overall performance. Building on extensive, in-depth understanding of feed additive applications, this new field is poised to help researchers explore innovative synergies between diverse product groups, such as enzymes, betaine, essential oils and probiotics. This is a challenging task as it goes beyond simply identifying which product groups work well together. It addresses the unique variances of each organism with the goal of understanding what specific organisms within each product do and how they work to complement each other.
When combined with proven data, this cutting-edge knowledge will enable the industry to learn how customised nutrition solutions can work within an animal’s own ecosystem to address common gut health issues, with the ultimate goal of increasing the health, wellness and productivity of livestock. An example of this research in today’s production environment is found in the unique enzyme and multi-strain probiotic solution of DuPont’s Syncra AVI and its impact on C.jejuni populations. Syncra AVI combines xylanase, amylase and protease enzymes with a unique combination of Bacillus probiotic strains. Individually:
-Xylanase breaks down the non-starch polysaccharides (NSPs) to release previously trapped nutrients ultimately reducing the levels of Campylobacter jejuni in the intestinal tract. The combined enzyme and probiotic mode of actions of Syncra AVI provide more available nutrients than single component additives, creating a more balanced gut microbiota.
-Amylase increases the hydrolysis of starch for improved digestibility
-Protease increases protein digestibility by hydrolysis of storage and structural proteins
-Bacillus probiotics help establish and maintain a beneficial microbial population in the gut, making it less conducive to colonisation by harmful microorganisms.
Multiple trials at independent research organisations have shown that the combination of these additives in one product works to improve performance from diets and reduce overall production costs, while also supporting gut health, ultimately reducing the levels of Campylobacter jejuni in the intestinal tract. The combined enzyme and probiotic mode of actions of Syncra AVI provide more available nutrients than single component additives, creating a more balanced gut microbiota.
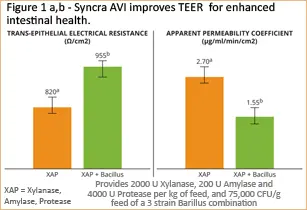 Demonstrating impact beyond basic measurements
Demonstrating impact beyond basic measurements
The enzymes and probiotics in Syncra AVI deliver complementary modes of action that provide more available nutrients than single component additives. In addition, they enable a more balanced gut microbiota, as shown through an improved gut structure. The intestinal tract is the largest interface between the body and the external environment. Intestinal permeability is a dynamic process being constantly shaped due to interactions with internal and external stimuli. One of the most common parameters to evaluate the intestinal permeability is trans-epithelial electrical resistance (TEER), which is the ability of semi-permeable tight junctions to differentially restrict free passage of water, ions and larger solutes based on size and charge, including bad bacteria. Syncra AVI was shown to improve TEER (and reduce permeability) when compared to enzymes alone (Figure 1).
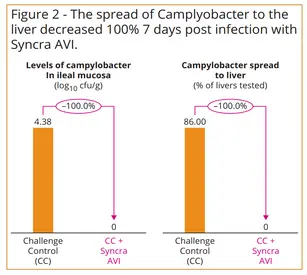 Applied, this means that Syncra AVI was also able to reduce the levels of Campylobacter jejuni in the intestinal tract and livers of challenged broilers fed a mixed grain diet. When fed at 200g/ton compared to the un-supplemented control, the product was shown to reduce the spread of C.jejuni to liver tissue (zero per cent positive post-enrichment) and reduce C.jejuni loads in the ileal mucosa (median of <1 log10 CFU/g) (Figure 2).
Applied, this means that Syncra AVI was also able to reduce the levels of Campylobacter jejuni in the intestinal tract and livers of challenged broilers fed a mixed grain diet. When fed at 200g/ton compared to the un-supplemented control, the product was shown to reduce the spread of C.jejuni to liver tissue (zero per cent positive post-enrichment) and reduce C.jejuni loads in the ileal mucosa (median of <1 log10 CFU/g) (Figure 2).
While there are multiple levels at which C.jejuni contamination can be targeted and interventions implemented, the reduction or elimination of C.jejuni in the poultry reservoirs is vital as the poultry intestine is the only amplification point for the pathogen throughout the food chain.
Improved production measures
In this new era of versatile measurements, the impact of improved intestinal health can be demonstrated through improvements in internal measurements, such as the TEER mentioned previously, as well as external production parameters such as footpad lesions scores. Compared to the challenged control, Syncra AVI was shown to numerically reduce footpad lesions by 16.2 per cent (Figure 3).
 Applying insight for production gains
Applying insight for production gains
The impact of this new and developing approach linking feed composition and a healthy gut function is clearly visible in the synergistic advantages of Syncra AVI. Through the exploration of innovative synergies between diverse product groups, producers will gain the knowledge necessary to make strategic feed decisions that improve performance and to maximise commercial return by unlocking the full potential of feed.



